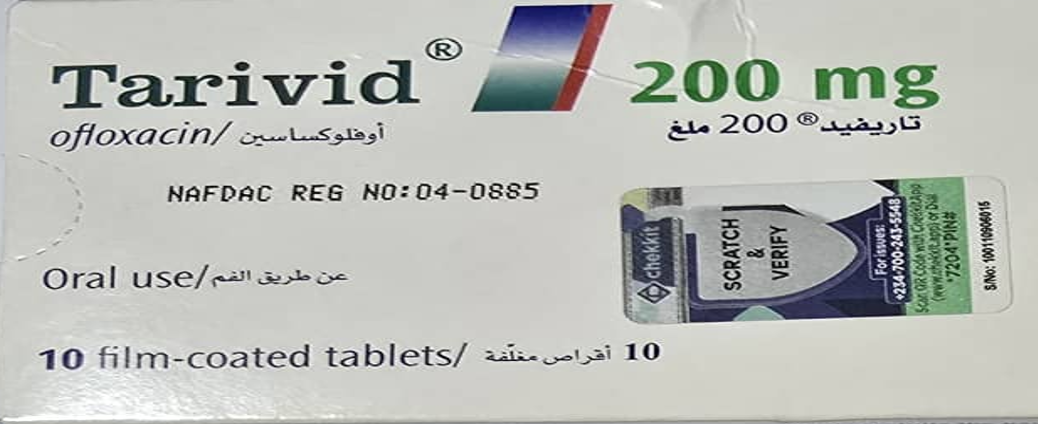
The National Agency for Food and Drug Administration and Control (NAFDAC) has raised an alarm over the circulation of an unregistered batch of Tarivid (Ofloxacin 200mg) tablets in Nigeria’s pharmaceutical supply chain. In a statement issued by the regulatory body, it confirmed that this specific batch of the antibacterial medication poses significant safety risks to public health and should be withdrawn immediately from all points of distribution.
NAFDAC disclosed that the affected batch of Tarivid was not duly registered in line with its regulatory requirements and, as such, has not undergone the necessary quality and safety checks mandated by the agency. Ofloxacin, the active ingredient in Tarivid, is a broad-spectrum antibiotic widely used to treat bacterial infections, including those of the respiratory tract, urinary tract, and skin. However, any formulation that has not passed through the agency’s stringent evaluation process can be dangerous for consumption.
According to NAFDAC, the product was discovered circulating within Nigeria’s healthcare system through routine surveillance and market intelligence operations carried out by its Investigation and Enforcement Directorate. The agency noted that the unregistered batch was being dispensed in both public and private health facilities as well as community pharmacies without authorization.
The Director-General of NAFDAC, Prof. Mojisola Adeyeye, reiterated the agency’s commitment to safeguarding the health of Nigerians, stressing that pharmaceutical products must be properly vetted before being allowed into the supply chain. She stated that NAFDAC will continue to intensify post-marketing surveillance activities across the country to detect and remove substandard and falsified medicines from circulation.
NAFDAC has urged healthcare providers, pharmacists, and the general public to exercise caution and report any suspicious pharmaceutical products to the nearest NAFDAC office or via the agency’s official channels. They also warned that anyone found in possession of or distributing the unregistered Tarivid product would face strict regulatory action.
Consumers are advised to check the authenticity of medicines by verifying the NAFDAC registration number and ensuring that packaging details match officially approved specifications. This development comes amid growing concerns over the infiltration of unregulated medical products into the Nigerian market, a problem that continues to threaten public safety and the integrity of the nation’s healthcare system.
As part of its response, NAFDAC has launched a full-scale investigation into how the unregistered batch entered the country and is working closely with relevant security and port authorities to trace its origin and prevent further distribution. The agency reaffirmed that only Tarivid products registered and certified by NAFDAC are safe for public use.
This warning serves as a critical reminder of the dangers posed by counterfeit and unregistered drugs and underscores the importance of strict regulatory compliance in the pharmaceutical industry.