The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert regarding the ban of certain Dove hand soaps and deodorants by European Union (EU) authorities. The affected products contain 2-(4-tert-butylbenzyl) propionaldehyde (BMHCA), a chemical prohibited in cosmetic products due to its potential to harm the reproductive system, affect unborn children, and cause skin sensitization.
Details of the Affected Products:
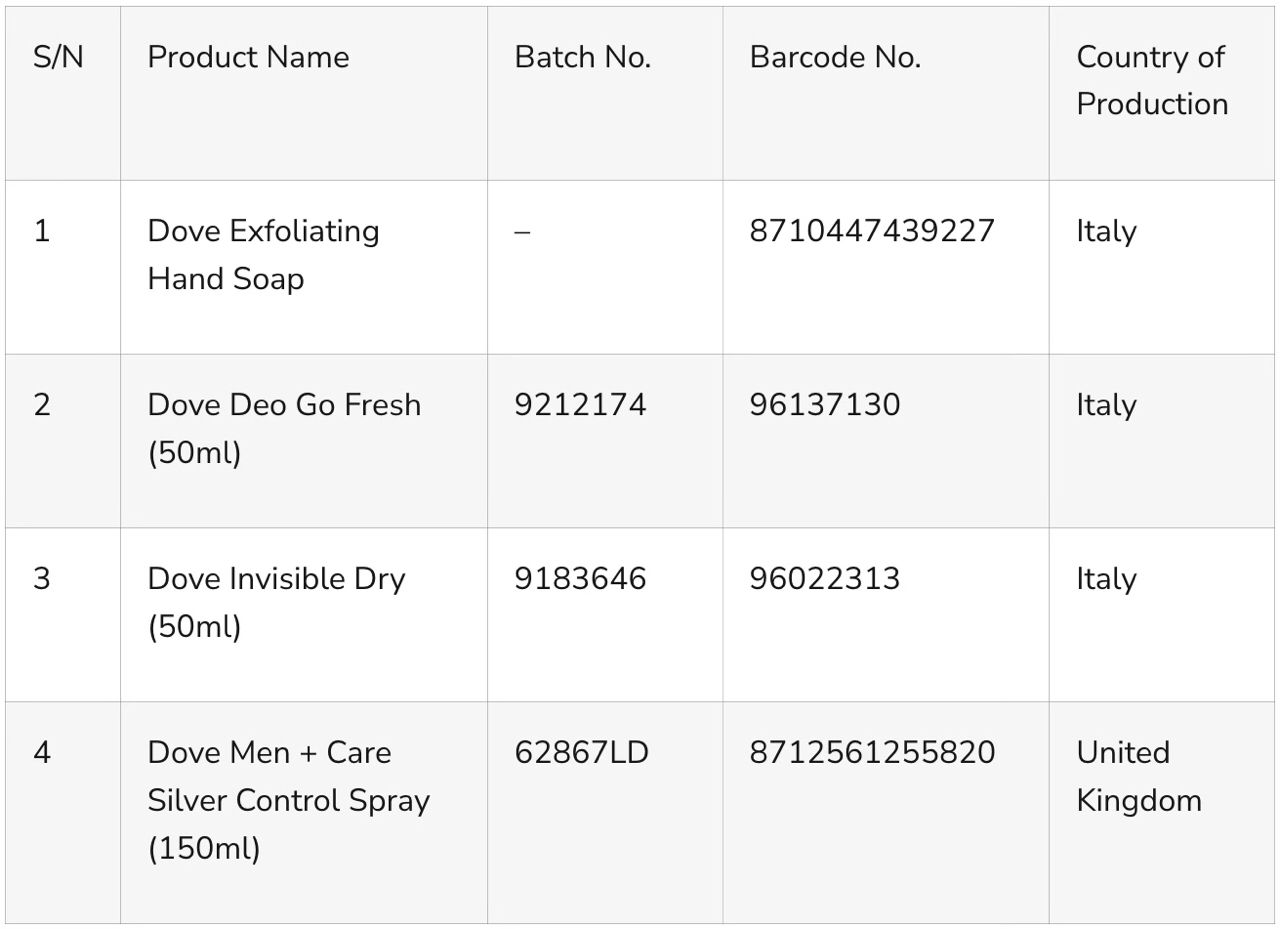
The following Dove products have been identified as containing BMHCA:
- Dove Exfoliating Hand Soap
Barcode: 8710447439227
Country of Manufacture: Italy
- Dove Deo Go Fresh (50ml)
Batch No.: 9212174
Barcode: 96137130
Country of Manufacture: Italy
- Dove Invisible Dry (50ml)
Batch No.: 9183646
Barcode: 96022313
Country of Manufacture: Italy
- Dove Men + Care Silver Control Spray (150ml)
Batch No.: 62867LD
Barcode: 8712561255820
Country of Manufacture: United Kingdom
These products have been banned in Brussels, Belgium, due to non-compliance with the EU’s Cosmetic Products Regulation. NAFDAC has confirmed that these products are not registered in its database and are therefore unauthorized for sale or distribution in Nigeria.
NAFDAC’s Advisory:
NAFDAC has advised importers, distributors, retailers, and consumers to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the above-mentioned products. Members of the public in possession of these products are urged to discontinue their use and submit the stock to the nearest NAFDAC office. Healthcare professionals and consumers are encouraged to report any adverse events experienced with the use of regulated products to NAFDAC via [email protected] or through the E-reporting platforms.