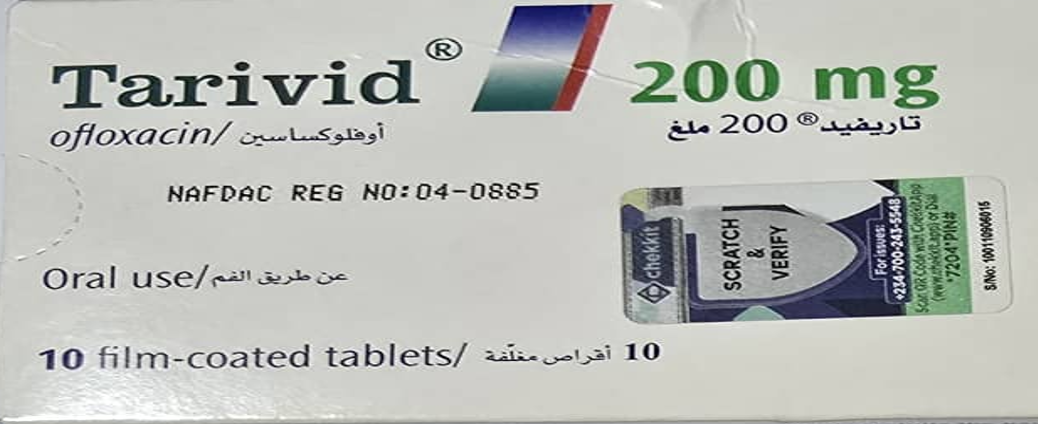
NAFDAC Intercepts Unregistered Batch of Tarivid, Warns of Public Health Risk
The National Agency for Food and Drug Administration and Control (NAFDAC) has raised an alarm over the circulation of an unregistered batch of Tarivid (Ofloxacin 200mg) tablets in Nigeria’s pharmaceutical supply chain. In a statement issued by the regulatory body, it confirmed that this specific batch of the antibacterial medication poses significant safety risks to